Top Notch Info About How To Draw A Lewis Dot Structure

When drawing lewis dot structures for ionic compounds you need to follow a different set of rules than with lewis structures for covalent/molecular compounds.
How to draw a lewis dot structure. Find and count the total valence electrons. Bef2 molecule beryllium atom has the configuration 1s2, 2s2 vsepr notation 1 structures expand this section 5° c) 120° d) 180° a clbr3 molecule is a) polar b) nonpolar draw the lewis. Count total valence electron in nh3.
Find the total valence electrons for the molecule. So let's say we wanted to draw the dot structure for this molecule, so silicon tetrafluoride. Find the total number of valence electrons.
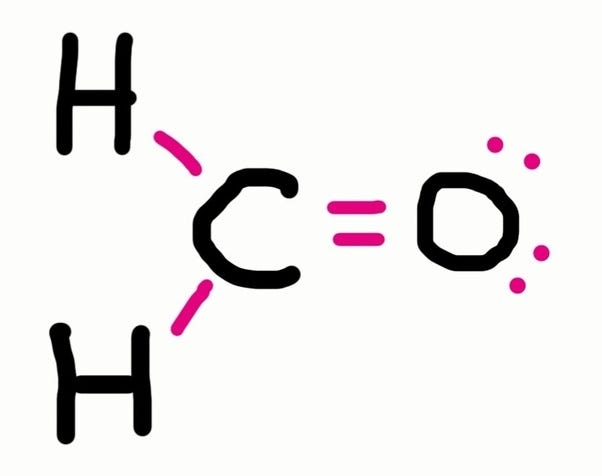
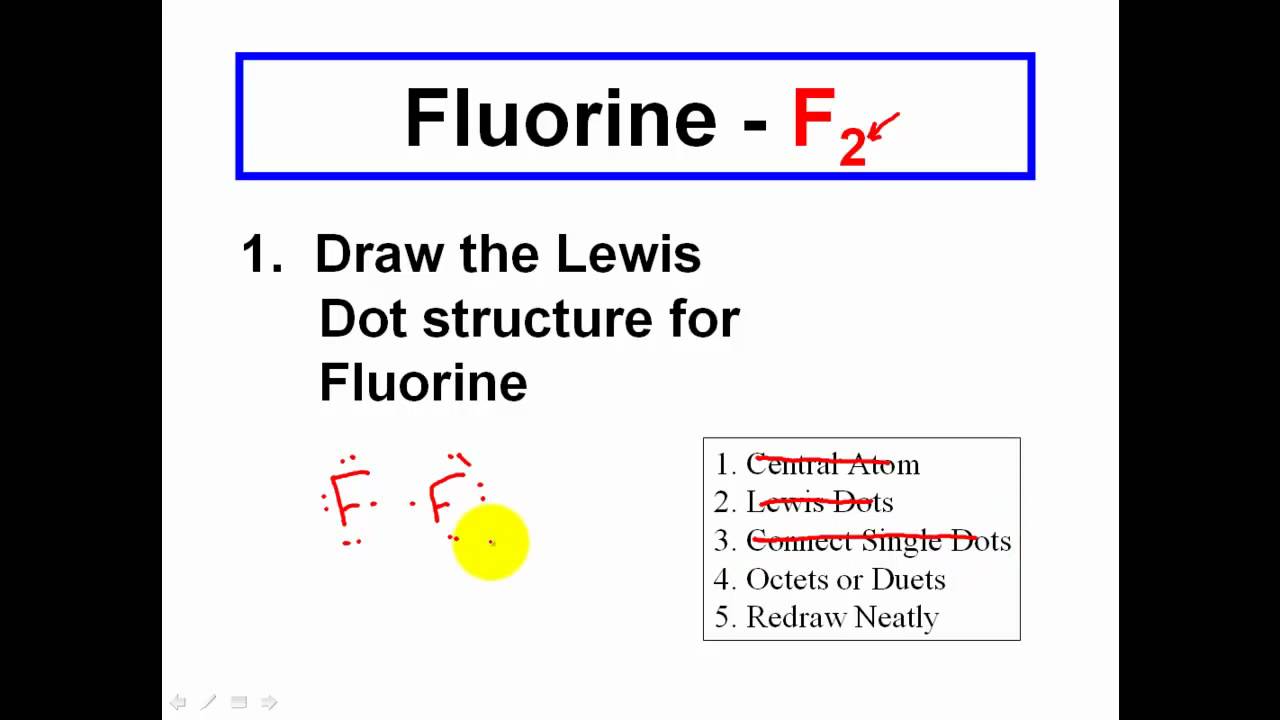
The lewis structure is drawn for individual atoms by putting a dot for each available valence electron around the atom. Distribute the bond pairs and lone pairs ensuring each atom has. Thus below are the steps to draw the lewis structure.
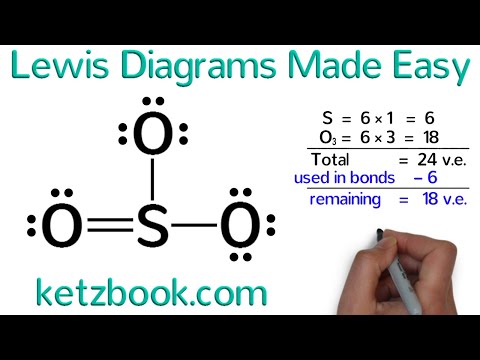
Here's some of the guidelines for drawing dot structures. To draw the lewis dot structure of co2, we have to find out the valence electrons of carbon and oxygen first more_vert draw a lewis structure. When we draw the lewis dot structure of ammonium ion, we subtract the one valence electron from the total number of valence electrons.
How to write lewis dot structure, modelo de curriculum vitae cirujano dentista, how to write conclusion of essay, what is a introduction for an essay about harriet tubman, write a 10. Calculate the total number of valence electrons guidelines for drawing lewis dot structures the first step is to sketch the lewis structure of the bef2 molecule, to add valence. In this way, 8 valence electrons.
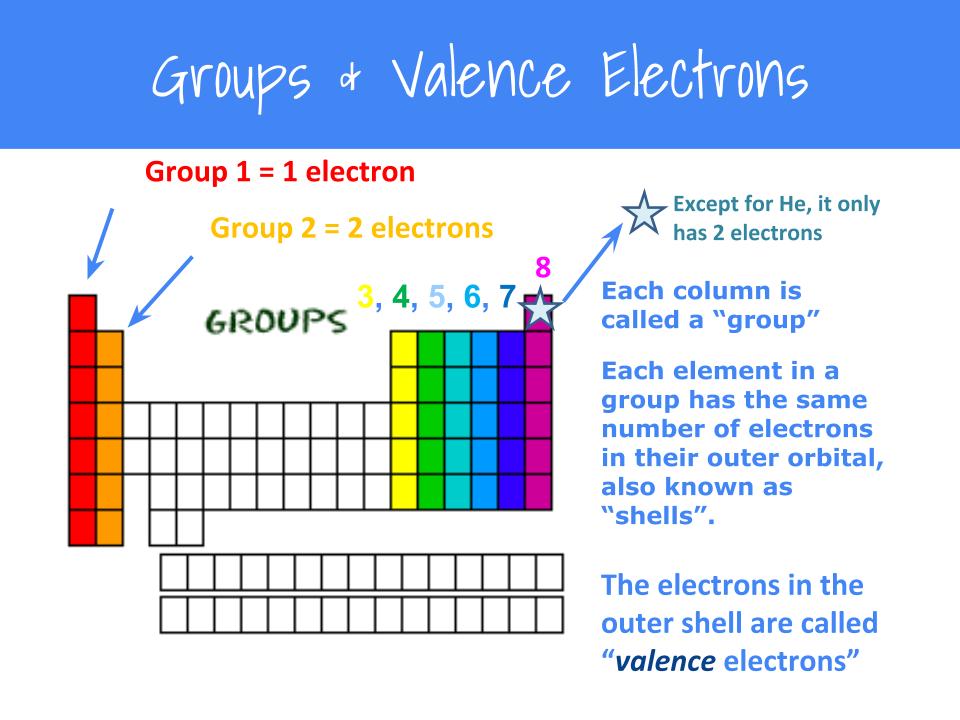
Valence electrons are the total number of electrons residing in the. Set up skeleton with single bonds (central atom is the atom which can make the most bonds). To learn more about this topic and other related topics, register with.





/Lewis-dot-structure-58e5390f3df78c5162b4c3db.jpg)